Published online Oct 14, 2022. doi: 10.3748/wjg.v28.i38.5602
Peer-review started: June 27, 2022
First decision: August 1, 2022
Revised: August 12, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: October 14, 2022
The optimal timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute cholangitis (AC) is uncertain, especially in patients with AC of varying severity.
To report whether the timing of ERCP is associated with outcomes in AC patients with different severities.
According to the 2018 Tokyo guidelines, 683 patients who met the definite diagnostic criteria for AC were retrospectively identified. The results were first compared between patients receiving ERCP ≤ 24 h and > 24 h and then between patients receiving ERCP ≤ 48 h and > 48 h. Subgroup analyses were performed in patients with grade I, II or III AC. The primary outcome was 30-d mortality. Secondary outcomes were intensive care unit (ICU) admission rate, length of hospital stay (LOHS) and 30-d readmission rate.
Taking 24 h as the critical value, compared with ERCP > 24 h, malignant biliary obstruction as a cause of AC was significantly less common in the ERCP ≤ 24 h group (5.2% vs 11.5%). The proportion of cardiovascular dysfunction (11.2% vs 2.6%), respiratory dysfunction (14.2% vs 5.3%), and ICU admission (11.2% vs 4%) in the ERCP ≤ 24 h group was significantly higher, while the LOHS was sign
ERCP ≤ 48 h conferred a survival benefit in patients with grade III AC. Early ERCP shortened the LOHS in patients with grade I and II AC.
Core Tip: Compared with endoscopic retrograde cholangiopancreatography (ERCP) > 24 h, ERCP ≤ 24 h group had a significantly higher intensive care unit (ICU) admission rate and shorter length of hospital stay (LOHS). Subgroup analysis showed higher ICU admission rate was only in grade III acute cholangitis (AC); shorter LOHS was only in grade II and I AC. Compared with ERCP > 48 h, ERCP ≤ 48 h group had significantly lower 30-d mortality and shorter LOHS. Subgroup analysis revealed lower 30-d mortality was only in grade III AC; shorter LOHS was only in grade II and I AC. We concluded that ERCP ≤ 48 h conferred a survival benefit in grade III AC; early ERCP shortened LOHS in grade II and I AC.
- Citation: Huang YC, Wu CH, Lee MH, Wang SF, Tsou YK, Lin CH, Sung KF, Liu NJ. Timing of endoscopic retrograde cholangiopancreatography in the treatment of acute cholangitis of different severity. World J Gastroenterol 2022; 28(38): 5602-5613
- URL: https://www.wjgnet.com/1007-9327/full/v28/i38/5602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i38.5602
Without treatment, patients with acute cholangitis (AC) may progress to septicemia and organ failure resulting in mortality[1,2]. Over the past two decades, endoscopic retrograde cholangiopancreatography (ERCP) has been generally accepted as a first-line treatment for AC[3,4]. Although delayed biliary drainage may not affect the risk of complications in patients who respond well to antibiotics, some patients with AC require early ERCP to avoid (persistent) organ failure or mortality[2,5,6]. Despite consensus on the need for biliary drainage, the optimal timing for early ERCP remains unclear due to mixed results in the literature[7]. Different definitions of early ERCP have been used in the literature, ranging from 12 h to 72 h[2,5,8-11]. The varied definitions among studies have led to inconsistent conclusions. In addition, the definitions of AC are not uniform across studies. More importantly, most studies did not define the timing of ERCP by stratifying the severity of AC[7]. In this context, the recently published Tokyo Guidelines 2018 (TG18) provide not only a diagnosis of AC but also a severity grading, which is important for predicting prognosis and determining treatment strategies[3]. However, although TG18 recommends early or urgent biliary drainage for moderate or severe cholangitis, there is no specific timing for early or urgent ERCP. Therefore, this study aims to investigate whether the timing of ERCP is associated with improved outcomes in AC patients with different severities.
This was a retrospective study conducted at Chang Gung Memorial Hospital Linkou Center. At our center, ERCP has been the first-line of treatment for patients with AC for the past two decades. This study was reviewed and approved by the Ethics Committee of the Chang Gung Memorial Hospital (IRB No. 202200881B0). Since this was a retrospective study using routine clinical treatment or diagnostic medical records, the Chang Gung Medical Foundation Institutional Review Board approved the waiver of the participant's consent. All methods were carried out under relevant guidelines and regulations.
The diagnostic criteria for AC were based on the 2018 Tokyo Guidelines, including systemic inflammation, cholestasis and imaging findings[3]. Systemic inflammation included fever (body temperature > 38 °C) or evidence of an inflammatory response [white blood cell (WBC) count < 4000 or > 10000/µL or C-reactive protein ≥ 1 mg/dL]. Cholestasis included jaundice (serum total bilirubin ≥ 2 mg/dL) or abnormal liver function tests (serum alkaline phosphatase, r-glutamyl transferase, aspartate aminotransferase, or alanine aminotransferase > 1.5 times the upper limit of the normal value). Imaging findings included bile duct dilatation or imaging evidence of etiology such as strictures, stones or stents. A definite diagnosis of AC was defined as one item in systemic inflammation, one item in cholestasis and one item in imaging findings.
AC severity was divided into three grades based on the 2018 Tokyo Guidelines[3]. Grade III (severe) AC was AC associated with the onset of dysfunction in at least one of the following organs/systems: cardiovascular dysfunction (defined as hypotension requiring dopamine ≥ 5 µg/kg per min, or any dose of norepinephrine), neurological dysfunction (presence of conscious disturbance), respiratory dysfunction (defined as PaO2/FiO2 ratio < 300), renal dysfunction (oliguria, serum creatinine > 2.0 mg/dL), hepatic dysfunction [defined as prothrombin time-international normalized ratio (PT-INR) > 1.5] or hematological dysfunction (defined as platelet count < 100 × 103/µL). Grade II (moderate) AC was AC associated with any two of the following conditions: abnormal WBC count (> 12000/µL or < 4000/µL), high fever (≥ 39 °C), old age (≥ 75 years), hyperbilirubinemia (serum total bilirubin ≥ 5 mg/dL), or hypoalbuminemia (< lower limit of normal value × 0.7). Grade I (mild) AC was AC that did not meet the criteria of “Grade III” or “Grade II” AC at initial diagnosis.
Time to ERCP was defined as the time from the emergency department visit to the commencement of ERCP.
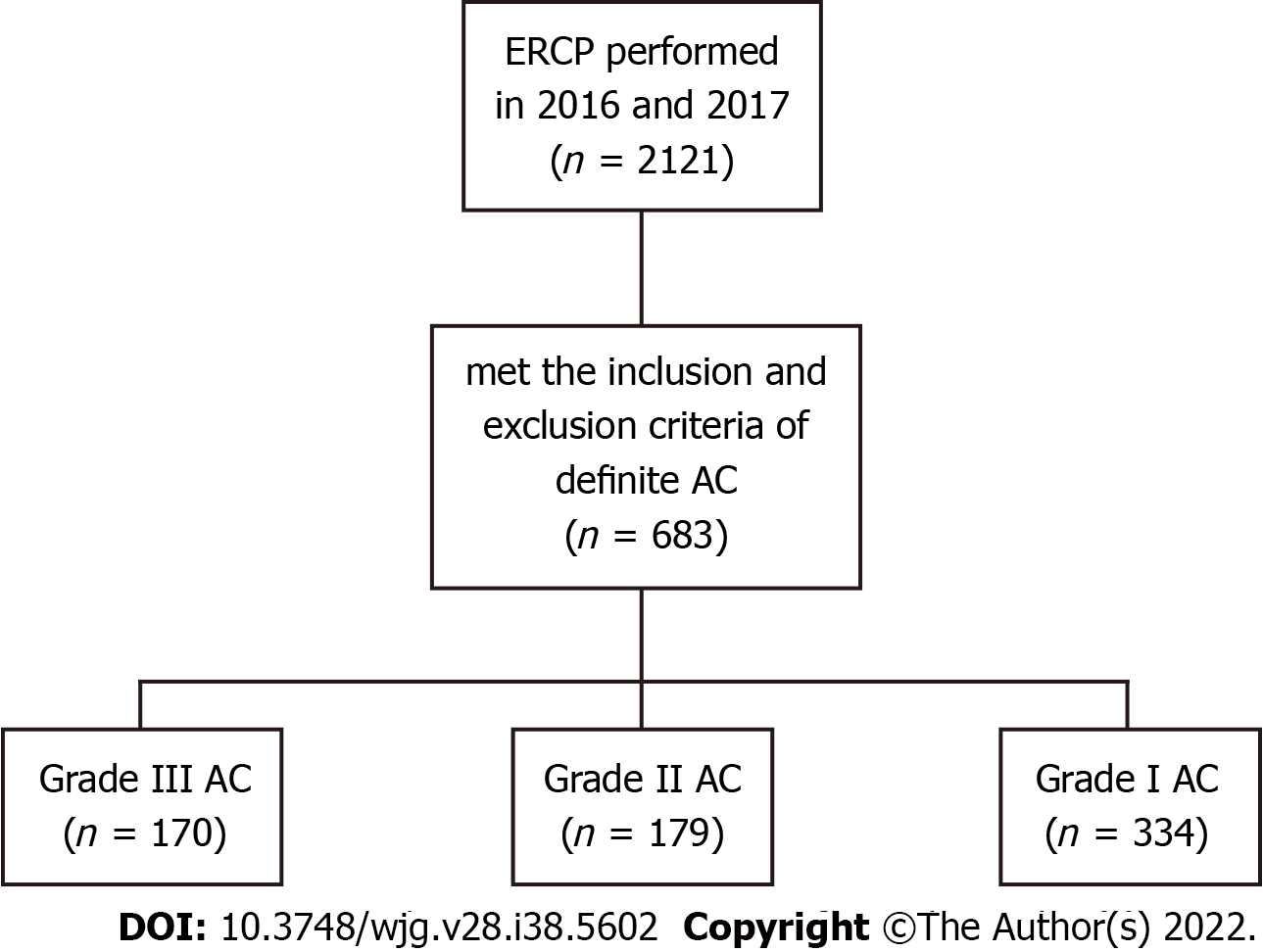
The study flow chart is shown in Figure 1. Between 2016 and 2017, 2121 patients who underwent ERCP in our center were retrospectively collected from the computer database of the Therapeutic Endoscopy Center. The inclusion criteria were patients who met the TG18/TG13 criteria for a definite diagnosis of AC. The exclusion criteria were: (1) Patients who did not meet the criteria for a definite diagnosis of AC; (2) inpatients who developed AC after hospitalization; and (3) patients who received ERCP 7 or more days after an emergency department visit. For patients readmitted for AC during the study period, we included only the first admission and the ERCP procedure.
Medical records were reviewed, and the following data were obtained: sex; age; clinical manifestations, including body temperature, systolic blood pressure, heart rate, saturation, respiratory rate and urine output; laboratory values including WBC count, platelet count, PT-INR, C-reactive protein, creatinine, bilirubin, alkaline phosphatase, r-glutamyl transferase, aspartate aminotransferase, alanine aminotransferase and albumin; diagnosis and treatment of ERCP, including causes of obstruction (such as stones, malignant strictures or stent dysfunction); and the timing of ERCP.
The primary outcome was 30-d mortality. Secondary outcomes were ICU admission rate, length of hospital stay (LOHS) and 30-d readmission rate. The results were first compared for patients receiving ERCP ≤ 24 h vs > 24 h and then for patients receiving ERCP ≤ 48 h vs > 48 h. Subgroup analyses were also performed in patients with grade I, II, and III AC.
Continuous variable data are represented by the median and interquartile range (IQR); categorical variables are presented as a number (%). For comparisons, the Kruskal-Wallis test was used for continuous variable data and the chi-square test or Fisher’s exact test was used for suitable categorical variables. Logistic regression analysis was performed to identify factors associated with 30-d mortality. Only variables with a P value < 0.05 in the univariate analysis were included in the multivariate analysis. Two-tailed P values < 0.05 were considered statistically significant and P values = 0.05 were considered marginally significant. Statistical analysis was performed using SPSS software (version 22.0; SPSS, Inc., Chicago, IL, United States).
A total of 683 patients who met the eligibility criteria were included in the study. Among them, there were 170 (24.9%) grade III AC patients, 179 grade II AC patients (26.4%) and 334 grade I AC patients (48.9%).
The baseline characteristics of the patients are presented in Table 1. The median (IQR) age of the patients was 66 (53-78) years; 57.2% were male. The median body temperature was 37.5 (36.8-38.4) °C and 58.4% of patients had abnormal WBC counts. The median platelet count was 198 × 103 (148 × 103-251 × 103)/µL. The median serum levels of aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase were 150 U/L, 166 U/L and 265 U/L, respectively. Serum amylase and lipase data were available for 307 (44.9%) and 487 (71.3%) patients with median levels of 65 U/L and 37 U/L, respectively. The median serum bilirubin level was 3.7 (2.3-6.3) mg/dL and the median creatine level was 0.93 (0.73-1.24) mg/dL. The median PT/INR was 1.1 (1.1-1.2). Only 133 (19.5%) patients had data on serum albumin and the median level was 3.56 (3.05-3.98) g/dL. Twenty-nine (4.2%) patients had cardiovascular dysfunction, 35 (5.1%) patients had neurological dysfunction and 48 (7%) patients had respiratory dysfunction.
| Patient characteristics | Total, n = 683 | Time to ERCP divided by 24 h | Time to ERCP divided by 48 h | ||||
| ERCP ≤ 24 h, n = 134 | ERCP > 24 h, n = 549 | P value | ERCP ≤ 48 h, n = 314 | ERCP > 48 h, n = 369 | P value | ||
| Age in yr1 | 66 (53-78) | 64 (56-75.5) | 66 (52-78) | 0.803 | 65 (54-79) | 66 (51-79) | 0.518 |
| Male sex, n (%) | 391 (57.2) | 76 (56.7) | 315 (57.4) | 0.890 | 171 (54.5) | 220 (59.6) | 0.174 |
| Body temperature in °C1 | 37.5 (36.8-38.4) | 37.2 (36.7-38.1) | 37.5 (36.9-38.5) | 0.003 | 37.4 (36.8-38.28) | 37.6 (36.9-38.5) | 0.009 |
| Abnormal WBC count2, n (%) | 399 (58.4) | 95 (70.9) | 304 (55.4) | 0.001 | 195 (62.1) | 204 (55.3) | 0.072 |
| Platelet count as /µL1 | 198 (148-251) | 205 (142-256) | 196.5 (149-250) | 0.806 | 195 (148-249.8) | 200 (148-251.5) | 0.844 |
| AST in U/L1 | 150 (83-305) | 175 (97-406) | 145 (80-289) | 0.063 | 164 (95-311) | 139 (76-302) | 0.08 |
| ALT in U/L1 | 166 (82-300) | 194 (118-337) | 156 (79-292) | 0.02 | 188 (101-311) | 142 (69-277) | 0.004 |
| ALK-P in U/L1 | 205 (133-327) | 198 (123-321) | 209 (133-327) | 0.486 | 190 (123-329) | 211 (140-324) | 0.208 |
| Amylase in U/L1 | 65 (41-170) (n = 307) | 68 (42-634) (n = 66) | 65 (40-145) (n = 241) | 0.126 | 68 (41-367) (n = 138) | 62 (40-139) (n = 169) | 0.118 |
| Lipase in U/L1 | 37 (26-144) (n = 487) | 46 (25-1297) (n = 97) | 36 (26-99) (n = 390) | 0.104 | 41 (26-339) (n = 232) | 35 (26-76) (n = 255) | 0.094 |
| Bilirubin in mg/dL1 | 3.7 (2.3-6.3) | 4.1 (2.6-5.8) | 3.7 (2.3-6.3) | 0.395 | 3.8 (2.4-6.2) | 3.7 (2.3-6.5) | 0.878 |
| PT/INR1 | 1.1 (1.1-1.2) | 1.1 (1.1-1.3) | 1.1 (1.1-1.2) | 0.685 | 1.1 (1.1-1.2) | 1.2 (1.1-1.3) | 0.001 |
| Creatinine in mg/dL1 | 0.93 (0.73-1.24) | 0.96 (0.78-1.45) | 0.93 (0.72-1.20) | 0.040 | 0.95 (0.75-1.28) | 0.93 (0.72-1.21) | 0.389 |
| Albumin in g/dL1 | 3.56 (3.05-3.98) (n = 133) | 3.83 (3.24-4.0) (n = 23) | 3.48 (3.05-3.98) (n = 110) | 0.218 | 3.69 (3.21-3.96) (n = 43) | 3.46 (2.98-3.99) (n = 90) | 0.334 |
| Cardiovascular dysfunction, n (%) | 29 (4.2) | 15 (11.2) | 14 (2.6) | < 0.001 | 18 (5.7) | 11 (3.0) | 0.076 |
| Neurological disturbance, n (%) | 35 (5.1) | 9 (6.7) | 26 (4.7) | 0.351 | 16 (5.1) | 19 (5.1) | 0.975 |
| Respiratory dysfunction, n (%) | 48 (7) | 19 (14.2) | 29 (5.3) | < 0.001 | 24 (7.6) | 24 (6.5) | 0.562 |
Comparisons between ERCP ≤ 24 h and ERCP > 24 h: Compared with ERCP > 24 h, patients with ERCP ≤ 24 h had significantly lower body temperature (median, 37.2 °C vs 37.5 °C. P = 0.003), significantly higher serum alanine aminotransferase (median, 194 U/L vs 156 U/L, P = 0.02) and serum creatinine levels (median, 0.96 vs 0.93, P = 0.004), and significantly higher proportions of abnormal WBC counts (70.9% vs 55.4%, P = 0.001), cardiovascular dysfunction (11.2% vs 2.6%, P < 0.001) and respiratory dysfunction (14.2% vs 5.3%, P < 0.001).
Comparisons between ERCP ≤ 48 h and ERCP > 48 h: Compared with ERCP > 48 h, patients with ERCP ≤ 48 h had significantly lower body temperature (median, 37.4 °C vs 37.6 °C, P = 0.009), significantly higher serum alanine aminotransferase levels (median, 188 U/L vs 142 U/L, P = 0.004) and significantly lower PT/INR (median, 1.1 vs 1.2, P = 0.001).
The characteristics of ERCP are listed in Table 2. Causes of AC included common bile duct stones (CBDS, 74.4%), malignant biliary obstruction (MBO, 10.2%), biliary stent dysfunction (8.9%), benign biliary stricture (4.5%) and others (1.9%). ERCP failed in 1% of patients. For patients with successful ERCP, endoscopic treatments during ERCP included endoscopic sphincterotomy (81.7%), endoscopic papillary balloon dilatation (0.7%), bile duct stone retrieval (73.5%), stone-free bile duct clearance (5.4%), removal of old biliary stents (8.8%), insertion of new biliary stents (27.4%), dilation of biliary strictures (1%) and others (0.4%).
| Patient characteristics | Total, n = 683 | Time to ERCP divided by 24 h | Time to ERCP divided by 48 h | ||||
| ERCP ≤ 24 h, n = 134 | ERCP > 24 h, n = 549 | P value | ERCP ≤ 48 h, n = 314 | ERCP > 48 h, n = 369 | P value | ||
| Time to ERCP in h1 | 53.8 (28.4-90.7) | 17.7 (9.0-20.4) | 67.6 (43.6-98.9) | - | 26.0 (18.8-40.1) | 88.5 (67.2-114.6) | |
| Indications of ERCP, n (%) | |||||||
| Common bile duct stones | 508 (74.4) | 107 (79.9) | 401 (73) | 0.106 | 256 (81.5) | 252 (68.3) | < 0.001 |
| Malignant obstruction | 70 (10.2) | 7 (5.2) | 63 (11.5) | 0.032 | 19 (6.1) | 51 (13.8) | < 0.001 |
| Stent dysfunction | 61 (8.9) | 8 (6.0) | 53 (9.7) | 0.180 | 17 (5.4) | 44 (11.9) | 0.003 |
| Benign stricture | 31 (4.5) | 8 (6.0) | 23 (4.2) | 0.375 | 15 (4.8) | 16 (4.3) | 0.783 |
| Others | 13 (1.9) | 4 (3.0) | 9 (1.6) | 0.296 | 7 (2.2) | 6 (1.6) | 0.565 |
| Treatment during ERCP, n (%) | |||||||
| ES | 558 (81.7) | 112 (83.6) | 446 (81.2) | 0.529 | 269 (85.7) | 289 (78.3) | 0.013 |
| EPBD | 5 (0.7) | 0 | 5 (0.9) | 0.589 | 2 (0.6) | 3 (0.8) | 1 |
| Stone retrieval | 502 (73.5) | 108 (80.6) | 394 (71.8) | 0.038 | 250 (79.6) | 252 (68.3) | 0.001 |
| Clearance without stone | 37 (5.4) | 5 (3.7) | 32 (5.8) | 0.336 | 16 (5.1) | 21 (5.7) | 0.732 |
| Removal of old stents | 60 (8.8) | 6 (4.5) | 54 (9.8) | 0.049 | 17 (5.4) | 43 (11.7) | 0.004 |
| Stent insertion | 187 (27.4) | 35 (26.1) | 152 (27.7) | 0.715 | 70 (22.3) | 117 (31.7) | 0.006 |
| Biliary stricture dilatation | 7 (1.0) | 1 (0.7) | 6 (1.1) | 1 | 1 (0.3) | 6 (1.6) | 0.132 |
| Others | 3 (0.4) | 0 | 3 (0.5) | 1 | 0 | 3 (0.8) | 0.254 |
| ERCP failure, n (%) | 7 (1.0) | 0 | 7 (1.3) | 0.356 | 1 (0.3) | 6 (1.6) | 0.132 |
Comparisons between ERCP ≤ 24 h and ERCP > 24 h: The median time to ERCP was 17.7 (9.0-20.4) h in the ERCP ≤ 24 h group and 67.6 (43.6-98.9) h in the ERCP > 24 h group. Only malignant biliary obstruction as a cause of AC was significantly less common in the ERCP ≤ 24 h group (5.2% vs 11.5%, P = 0.032). In therapeutic ERCP, bile duct stone retrieval was higher in the ERCP ≤ 24 h group (80.6% vs 71.8%, P = 0.038) whereas the old biliary stent removal rate was lower (4.5% vs 9.8%, P = 0.049).
Comparisons between ERCP ≤ 48 h and ERCP > 48 h: The median time to ERCP was 26.0 (18.8-40.1) h in the ERCP ≤ 48 h group and 88.5 (67.2-114.6) h in the ERCP > 48 h group. Regarding indications for ERCP, CBDS was more common in the ERCP ≤ 48 h group (81.5% vs 68.3%, P < 0.001) whereas malignant biliary obstruction (6.1% vs 13.8%, P < 0.001) and stent dysfunction were less common (5.4% vs 11.9%, P = 0.003). In therapeutic ERCP, endoscopic sphincterotomy (85.7% vs 78.3%, P = 0.013) and bile duct stone retrieval (79.6% vs 68.3%, P = 0.001) were more frequent in the ERCP ≤ 48 h group whereas the removal of old biliary stents (5.4% vs 11.7%, P = 0.004) and the insertion of new biliary stents (22.3% vs 31.7%, P = 0.006) were less frequent.
The primary and secondary outcomes are summarized in Table 3.
| Outcomes | Total | Time to ERCP divided by 24 h | Time to ERCP divided by 48 h | ||||
| ERCP ≤ 24 h | ERCP > 24 h | P value | ERCP ≤ 48 h | ERCP > 48 h | P value | ||
| Overall | n = 683 | n = 134 | n = 549 | n = 314 | n = 369 | ||
| 30-d mortality, n (%) | 7 (1.02) | 0 | 7 (1.3) | 0.356 | 0 | 7 (1.9) | 0.017 |
| ICU admission, n (%) | 37 (5.4) | 15 (11.2) | 22 (4) | 0.001 | 22 (7.0) | 15 (4.07) | 0.091 |
| LOHS in d1 | 7 (5-10) | 6 (4-10) | 7 (5-10) | 0.018 | 6 (4-9) | 8 (6-11) | < 0.001 |
| 30-d readmission, n (%) | 87 (12.7) | 16 (11.9) | 71 (12.9) | 0.757 | 40 (12.7) | 47 (12.7) | 0.999 |
| Grade III AC | n = 170 | n = 39 | n = 131 | n = 72 | n = 98 | ||
| 30-d mortality, n (%) | 6 (3.5) | 0 | 6 (4.6) | 0.338 | 0 | 6 (6.1) | 0.039 |
| ICU admission, n (%) | 26 (15.3) | 12 (9.0) | 14 (2.6) | 0.002 | 16 (22.2) | 10 (10.2) | 0.031 |
| LOHS in d1 | 7 (7-14) | 10 (6-16.5) | 9 (7-12) | 0.637 | 9 (6-15) | 9 (7-12) | 0.448 |
| 30-d readmission, n (%) | 23 (13.5) | 4 (3) | 19 (14.5) | 0.602 | 10 (13.9) | 13 (13.3) | 0.906 |
| Grade II AC | n = 179 | n = 39 | n = 140 | n = 88 | n = 91 | ||
| 30-d mortality, n (%) | 0 | 0 | 0 | - | 0 | 0 | - |
| ICU admission, n (%) | 5 (2.8) | 2 (5.1) | 3 (2.1) | 0.299 | 3 (3.4) | 2 (2.2) | 0.679 |
| LOHS in d1 | 7 (5-10) | 6 (4-9.5) | 7 (5.8-10) | 0.047 | 6 (4.8-9) | 8 (6-11) | 0.001 |
| 30-d readmission, n (%) | 24 (13.4) | 4 (10.3) | 20 (14.3) | 0.514 | 8 (9.1) | 16 (17.6) | 0.096 |
| Grade I AC | n = 334 | n = 56 | n = 278 | n = 154 | n = 180 | ||
| 30-d mortality, n (%) | 1 (0.3) | 0 | 1 (0.36) | 1 | 0 | 1 (0.56) | 1 |
| ICU admission, n (%) | 6 (1.8) | 1 (1.8) | 5 (1.8) | 1 | 3 (1.9) | 3 (1.7) | 1 |
| LOHS in d1 | 6 (5-9) | 6 (4-8.3) | 7 (5-9.8) | 0.005 | 6 (4-7) | 7 (6-11) | < 0.001 |
| 30-d readmission, n (%) | 40 (12) | 8 (14.3) | 32 (11.5) | 0.56 | 22 (14.3) | 18 | 0.229 |
Overall patients: Overall, the 30-d mortality rate was 1.02% (or 7/683). The ICU admission rate was 5.4%; the median LOHS was 7 (5-10) d; and the 30-d readmission rate was 12.7%.
(1) Comparisons between ERCP ≤ 24 h and ERCP > 24 h: The overall 30-d mortality rate was 0 in the ERCP ≤ 24 h group and 1.3% in the ERCP > 24 h group. However, the difference did not reach statistical significance (P = 0.356). Regarding secondary outcomes, the ERCP ≤ 24 h group had significantly higher ICU admission rates (11.2% vs 4.0%, P = 0.001) and shorter LOHS (median, 6 d vs 7 d, P = 0.018).
(2) Comparisons between ERCP ≤ 48 h and ERCP > 48 h: Overall, the 30-d mortality rate was significantly lower in the ERCP ≤ 48 h group than in the ERCP > 48 h group (0 vs 1.9%, P = 0.017). For secondary outcomes, the ERCP ≤ 48 h group had a significantly shorter LOHS (median, 6 d vs 8 d, P < 0.001).
Patients with Grade III AC: The 30-d mortality rate was 3.5% (or 6/170) for patients with grade III AC. The ICU admission rate was 15.3%; the median LOHS was 7 (7-14) d; and the 30-d readmission rate was 13.5% in this patient group.
(1) Comparisons between ERCP ≤ 24 h and ERCP > 24 h: The 30-d mortality rate for grade III AC patients was 0 in the ERCP ≤ 24 h group and 4.6% in the ERCP > 24 h group. However, the difference did not reach statistical significance (P = 0.338). Regarding secondary outcomes, the ERCP ≤ 24 h group had significantly higher ICU admission rates (9.0% vs 2.6%, P = 0.002).
(2) Comparisons between ERCP ≤ 48 h and ERCP > 48 h: Among grade III AC patients, the 30-d mortality rate was significantly lower in the ERCP ≤ 48 h group than in the ERCP > 48 h group (0 vs 6.1%, P = 0.039). Regarding secondary outcomes, the ERCP ≤ 48 h group had significantly higher ICU admission rates (22.2% vs 10.2%, P = 0.031).
Patients with Grade II AC: The 30-d mortality rate was 0 for patients with grade II AC. The ICU admission rate was 2.8%; the median LOHS was 7 (5-10) d; and the 30-d readmission rate was 13.4% in this patient group.
(1) Comparisons between ERCP ≤ 24 h and ERCP > 24 h: The only significant finding in grade II AC patients was that the LOHS was shorter (median, 6 d vs 7 d, P = 0.047) in the ERCP ≤ 24 h group.
(2) Comparisons between ERCP ≤ 48 h and ERCP > 48 h: Among grade II AC patients, the only significant finding was that the LOHS was shorter (median, 6 d vs 8 d, P = 0.001) in the ERCP ≤ 48 h group.
Patients with Grade I AC: The 30-d mortality rate was 0.3% (or 1/334) for patients with grade I AC. The ICU admission rate was 1.8%; the median LOHS was 6 (5-9) d; and the 30-d readmission rate was 12% in this patient group.
(1) Comparisons between ERCP ≤ 24 h and ERCP > 24 h: The only significant finding in grade I AC patients was that the LOHS was shorter (median, 6 d vs 7 d, P = 0.005) in the ERCP ≤ 24 h group.
(2) Comparisons between ERCP ≤ 48 h and ERCP > 48 h: Among grade I AC patients, the only significant finding was that the LOHS was shorter (median, 6 d vs 7 d, P < 0.001) in the ERCP ≤ 48 h group.
The results of the univariate and multivariate analyses are listed in Table 4. The univariate analysis revealed that malignant biliary obstruction (OR: 6.817, 95%CI: 1.494-31.109, P = 0.013), hepatic dysfunction (OR: 8.896, 95%CI: 1.645-48.119, P = 0.011), respiratory dysfunction (OR: 10.517, 95%CI: 2.284-48.431, P = 0.003), neurological dysfunction (OR: 15.094, 95%CI: 3.241-70.298, P = 0.001), cardiovascular dysfunction (OR: 18.750, 95%CI: 3.990-88.112, P < 0.001), severity of AC (severe vs moderate + mild, OR: 18.732, 95%CI: 2.239-156.728, P = 0.007), ICU admission (OR: 7.326, 95%CI: 1.373-39.101, P = 0.02), and time to ERCP (every 1-d delay, OR: 1.950, 95%CI: 1.252-3.038, P = 0.003) were associated with 30-d mortality. The multivariate analysis revealed that time to ERCP (every 1-d delay, OR: 2.081, 95%CI: 1.154-3.753, P = 0.015) was the only independent factor associated with 30-d mortality. However, cardiovascular dysfunction (OR: 17.756, 95%CI: 0.994-317.241, P = 0.050) was of marginal significance.
| Variables | Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age | ≥ 75 yr | 1.772 (0.393-7.991) | 0.456 | ||
| < 75 yr | |||||
| Reference | |||||
| Common bile duct stones | Yes | 0.254 (0.056-1.146) | 0.075 | ||
| No | |||||
| Reference | |||||
| Malignant biliary obstruction | Yes | 6.817 (1.494-31.109) | 0.013 | 7.718 (0.664-89.660) | 0.102 |
| No | |||||
| Reference | |||||
| Fever, BT ≥ 39 °C | Yes | 1.045 (0.124-8.777) | 0.968 | ||
| No | |||||
| Reference | |||||
| Abnormal WBC count | Yes | 1.789 (0.345-9.289) | 0.489 | ||
| No | |||||
| Reference | |||||
| Hyperbilirubinemia, ≥ 5 mg/dL | Yes | 1.382 (0.307-6.228) | 0.673 | ||
| No | |||||
| Reference | |||||
| Hepatic dysfunction, PT-INR > 1.5 | Yes | 8.896 (1.645-48.119) | 0.011 | 2.257 (0.275-18.553) | 0.449 |
| No | |||||
| Reference | |||||
| Hematological dysfunction, PLT < 100 × 103/µL | Yes | 4.885 (0.919-25.649) | 0.063 | ||
| No | |||||
| Reference | |||||
| Renal dysfunction, Cr > 2.0 mg/dL | Yes | 4.548 (0.862-23.998) | 0.074 | ||
| No | |||||
| Reference | |||||
| Respiratory dysfunction, PaO2/FiO2 ratio > 300 | Yes | 10.517 (2.284-48.431) | 0.003 | 1.644 (0.172-15.676) | 0.666 |
| No | |||||
| Reference | |||||
| Neurological dysfunction, conscious disturbance | Yes | 15.094 (3.241-70.298) | 0.001 | 2.773 (0.380-20.238) | 0.315 |
| No | |||||
| Reference | |||||
| Cardiovascular dysfunction1 | Yes | 18.750 (3.990-88.112) | < 0.001 | 17.756 (0.994-317.241) | 0.050 |
| No | |||||
| Reference | |||||
| Severity of AC | Grade III | 18.732 (2.239-156.728) | 0.007 | 3.603 (0.274-47.356) | 0.329 |
| Grade II + I | |||||
| Reference | |||||
| ICU admission | Yes | 7.326 (1.373-39.101) | 0.02 | 2.463 (0.204-29.703) | 0.478 |
| No | |||||
| Reference | |||||
| Time to ERCP | Every 1-d delay | 1.950 (1.252-3.038) | 0.003 | 2.081 (1.154-3.753) | 0.015 |
Kiriyama et al[3] reported that early or urgent ERCP significantly reduced 30-d mortality only in patients with grade II AC compared with patients who did not receive early or urgent ERCP. This result may be due to the lack of well-defined timing for early or urgent ERCP. In a meta-analysis published in 2020, Du et al[7] reported that early ERCP reduced in-hospital mortality regardless of whether it was defined as < 24 h, < 48 h or < 72 h. In the present study, we found that ERCP ≤ 48 h but not ERCP ≤ 24 h significantly reduced 30-d mortality. Our results were consistent with the 2021 American Society for Gastrointestinal Endoscopy guidelines recommending ERCP ≤ 48 h in AC patients[12]. In that study, however, the data were insufficient to stratify by disease severity. In a subgroup analysis, we found that the same survival benefit was observed only in patients with grade III AC but not in patients with grade II or I AC. These results were because patients with grade III AC had significantly higher 30-d mortality than those with grade II or I AC (3.5% vs 0 vs 0.3%, P = 0.001). Hakuta et al[10] reported that time to ERCP was not associated with clinical outcomes (including in-hospital mortality) in patients with non-grade III AC. Therefore, we recommend emergent ERCP (≤ 48 h) for patients with grade III AC in terms of survival benefit.
However, 30-d mortality in AC has been reported to range from 1% to 16% between studies, which may be one of the reasons leading to inconsistent conclusions about the optimal timing of ERCP[11,13-15]. Differences in mortality may be due to different patient populations in different studies, e.g., patients with AC due to CBDS and MBO may have different clinical courses and prognoses. Kiriyama et al[14] reported that patients with AC associated with MBO had a higher 30-d mortality rate than those with AC associated with CBDS. In our univariate analysis, MBO was a factor associated with 30-d mortality. Therefore, one of the reasons for the low 30-d mortality in our study was the low proportion of patients with MBO (10.2%). In contrast, Tan et al[11] included 43% of MBO patients in their study and reported a 30-d mortality rate of 16%. However, Park et al[15] included only patients with AC associated with distal MBO and reported an overall 30-d mortality rate of 4.8%. Therefore, there may be some other factors associated with 30-d mortality between studies.
Of the five organ failure criteria used to diagnose grade III AC, cardiovascular dysfunction was the only independent factor associated with 30-d mortality in the current study. Therefore, among grade III AC patients, those with cardiovascular dysfunction may need to be treated differently[16,17]. Karvellas et al[17] reported an overall mortality rate of 37% in 260 patients with AC-related septic shock. They found that delayed biliary decompression > 12 h from the onset of shock was one of three independent factors associated with mortality. The 2019 European Society of Gastrointestinal Endoscopy guidelines recommend biliary drainage (preferably endoscopic) within 12 h of shock onset for AC patients with CBDS-related septic shock[16]. Therefore, cardiovascular dysfunction should be weighed when developing new guidelines in the future.
Some studies found no survival benefit but did find early ERCP to reduce the LOHS[18-21]. Hou et al[20] reported that in a multivariate analysis, the LOHS increased by 1.44 d for every 1-d delay in ERCP (P < 0.001). Similar results were seen in the study by Zhu et al[18]: LOHS increased by 1.49 d for every 1-d delay in biliary drainage (P < 0.0001). However, these findings were not stratified by disease severity. Although we did not perform a multivariate analysis of the LOHS, our results suggested that the LOHS could be significantly reduced regardless of ERCP ≤ 24 h or ≤ 48 h. In subgroup analyses stratified by disease severity, this benefit was only observed in patients with grade I or II AC. The benefit of early ERCP in shortening the LOHS might be offset by higher ICU admission rates in grade III AC patients. Similar findings were found by Jang et al[19], who recommended urgent ERCP (≤ 24 h) for patients with grade I or II AC because it can shorten the LOHS.
This study has several limitations. First, this retrospective, single-center study might have inherent selection bias. Patients with cardiovascular dysfunction and respiratory dysfunction tended to receive ERCP ≤ 24 h. Therefore, caution is required when interpreting the results of this subgroup analysis. Second, we identified patients from the endoscopy database. AC patients who died before receiving ERCP might not have been included in this study, resulting in an underestimation of 30-d mortality. Third, data on albumin, one of the criteria for class II AC, were available in only 19.5% of patients. Therefore, some patients with grade II AC may be misclassified as grade I AC and vice versa.
ERCP ≤ 48 h but not ≤ 24 h has a survival benefit in AC patients; this benefit is only observed in patients with grade III AC. Early ERCP is also recommended for patients with grade I or II AC because it shortens the LOHS.
The optimal timing of endoscopic retrograde cholangiopancreatography (ERCP) for acute cholangitis has been inconsistently reported and there are few studies on the timing of ERCP in acute cholangitis of varying severity.
On the one hand, unnecessary emergent ERCP increases medical costs and the burden on physicians and technicians; on the other hand, delayed ERCP may increase morbidity and mortality in patients with acute cholangitis. The findings of this study may guide the avoidance of unnecessary urgent and delayed ERCP for acute cholangitis.
This study aims to answer the optimal timing of ERCP for acute cholangitis of different severity according to 30-d mortality after ERCP. Answering this question can serve as important evidence for future guideline development.
The retrospective cohort study included 683 patients who met the diagnostic criteria for acute cholangitis defined by the 2018 Tokyo Guidelines. Among them, there were 170 (24.9%) grade III acute cholangitis patients, 179 grade II acute cholangitis patients (26.4%) and 334 grade I acute cholangitis patients (48.9%). Results are first compared between patients receiving ERCP ≤ 24 h and > 24 h, and then between patients receiving ERCP ≤ 48 h and > 48 h. Subgroup analyses are performed on patients with grade III, II or I acute cholangitis.
When 24 h was considered a critical value for ERCP timing, we found that patients with malignant biliary obstruction received ERCP ≤ 24 h less frequently when compared with ERCP > 24 h (5.2% vs 11.5%). Patients with organ dysfunction such as cardiovascular dysfunction (11.2% vs 2.6%) and respiratory dysfunction (14.2% vs 5.3%) or those admitted to the ICU (11.2% vs 4%) tended to receive ERCP ≤ 24 h. Patients with ERCP ≤ 24 h had significantly shorter hospital stays (median, 6 d vs 7 d). Stratified by the severity of acute cholangitis, higher ICU admission was only observed in grade III acute cholangitis and a shorter length of hospital stay was only observed in grade I and II acute cholangitis. Regarding 30-d mortality, the results of ERCP ≤ 24 h and > 24 h were not significantly different, either in the overall population or in patients with grade I, II or III acute cholangitis. When 48 h was considered a critical value for ERCP timing, patients with choledocholithiasis received ERCP ≤ 48 h more frequently (81.5% vs 68.3%). Patients who received ERCP ≤ 48 h had significantly lower 30-d mortality (0 vs 1.9%) and shorter hospital stays (6 d vs 8 d). Stratified by the severity of acute cholangitis, lower 30-d mortality (0 vs 6.1%) and higher ICU admission rates (22.2% vs 10.2%) were only observed in grade III acute cholangitis and a shorter length of hospital stay was only observed in grade I and II acute cholangitis. In the multivariate analysis, cardiovascular dysfunction and time to ERCP were two independent factors associated with 30-d mortality.
ERCP ≤ 48 h but not ≤ 24 h has a survival benefit in acute cholangitis patients; this benefit is only observed in patients with grade III acute cholangitis. Early ERCP is also recommended for patients with grade I and II acute cholangitis because it shortens the length of hospital stay.
Of the five organ failure criteria used to diagnose grade III AC, cardiovascular dysfunction was the only independent factor associated with 30-d mortality in the current study. Therefore, cardiovascular dysfunction should be weighed more heavily in the development of new guidelines in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isogai M, Japan; Liao JX, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Mosler P. Diagnosis and management of acute cholangitis. Curr Gastroenterol Rep. 2011;13:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 2. | Lee F, Ohanian E, Rheem J, Laine L, Che K, Kim JJ. Delayed endoscopic retrograde cholangiopancreatography is associated with persistent organ failure in hospitalised patients with acute cholangitis. Aliment Pharmacol Ther. 2015;42:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 4. | Xu MM, Carr-Locke DL. Early ERCP for severe cholangitis? Gastrointest Endosc. 2018;87:193-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Khashab MA, Tariq A, Tariq U, Kim K, Ponor L, Lennon AM, Canto MI, Gurakar A, Yu Q, Dunbar K, Hutfless S, Kalloo AN, Singh VK. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10:1157-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Iqbal U, Khara HS, Hu Y, Khan MA, Ovalle A, Siddique O, Sun H, Shellenberger MJ. Emergent versus urgent ERCP in acute cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91:753-760.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 7. | Du L, Cen M, Zheng X, Luo L, Siddiqui A, Kim JJ. Timing of Performing Endoscopic Retrograde Cholangiopancreatography and Inpatient Mortality in Acute Cholangitis: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2020;11:e00158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Navaneethan U, Gutierrez NG, Jegadeesan R, Venkatesh PG, Sanaka MR, Vargo JJ, Parsi MA. Factors predicting adverse short-term outcomes in patients with acute cholangitis undergoing ERCP: A single center experience. World J Gastrointest Endosc. 2014;6:74-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Nishino T, Hamano T, Mitsunaga Y, Shirato I, Shirato M, Tagata T, Shimada M, Yoshida S, Mitsunaga A. Clinical evaluation of the Tokyo Guidelines 2013 for severity assessment of acute cholangitis. J Hepatobiliary Pancreat Sci. 2014;21:841-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Hakuta R, Hamada T, Nakai Y, Kogure H, Uchino R, Takahara N, Mizuno S, Suzuki T, Sato T, Takeda T, Ishigaki K, Saito K, Saito T, Tada M, Isayama H, Koike K. No Association of Timing of Endoscopic Biliary Drainage with Clinical Outcomes in Patients with Non-severe Acute Cholangitis. Dig Dis Sci. 2018;63:1937-1945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Tan M, Schaffalitzky de Muckadell OB, Laursen SB. Association between early ERCP and mortality in patients with acute cholangitis. Gastrointest Endosc. 2018;87:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Buxbaum JL, Buitrago C, Lee A, Elmunzer BJ, Riaz A, Ceppa EP, Al-Haddad M, Amateau SK, Calderwood AH, Fishman DS, Fujii-Lau LL, Jamil LH, Jue TL, Kwon RS, Law JK, Lee JK, Naveed M, Pawa S, Sawhney MS, Schilperoort H, Storm AC, Thosani NC, Qumseya BJ, Wani S. ASGE guideline on the management of cholangitis. Gastrointest Endosc. 2021;94:207-221.e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | On W, Watters C, Dwyer L, Hood S, Saleem R, Sturgess R, Stern N. P55 Timing of ERCP and outcomes in patients with acute gallstone cholangitis graded by severity. Gut. 2021;70 (Suppl 1):A69. [DOI] [Cited in This Article: ] |
| 14. | Kiriyama S, Takada T, Hwang TL, Akazawa K, Miura F, Gomi H, Mori R, Endo I, Itoi T, Yokoe M, Chen MF, Jan YY, Ker CG, Wang HP, Wada K, Yamaue H, Miyazaki M, Yamamoto M. Clinical application and verification of the TG13 diagnostic and severity grading criteria for acute cholangitis: an international multicenter observational study. J Hepatobiliary Pancreat Sci. 2017;24:329-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Park N, Lee SH, You MS, Kim JS, Huh G, Chun JW, Cho IR, Paik WH, Ryu JK, Kim YT. Optimal timing of endoscopic retrograde cholangiopancreatography for acute cholangitis associated with distal malignant biliary obstruction. BMC Gastroenterol. 2021;21:175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, Barthet M, Domagk D, Dumonceau JM, Gigot JF, Hritz I, Karamanolis G, Laghi A, Mariani A, Paraskeva K, Pohl J, Ponchon T, Swahn F, Ter Steege RWF, Tringali A, Vezakis A, Williams EJ, van Hooft JE. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 286] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 17. | Karvellas CJ, Abraldes JG, Zepeda-Gomez S, Moffat DC, Mirzanejad Y, Vazquez-Grande G, Esfahani EK, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. The impact of delayed biliary decompression and anti-microbial therapy in 260 patients with cholangitis-associated septic shock. Aliment Pharmacol Ther. 2016;44:755-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Zhu Y, Tu J, Zhao Y, Jing J, Dong Z, Pan W. Association of Timing of Biliary Drainage with Clinical Outcomes in Severe Acute Cholangitis: A Retrospective Cohort Study. Int J Gen Med. 2021;14:2953-2963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Jang SE, Park SW, Lee BS, Shin CM, Lee SH, Kim JW, Jeong SH, Kim N, Lee DH, Park JK, Hwang JH. Management for CBD stone-related mild to moderate acute cholangitis: urgent versus elective ERCP. Dig Dis Sci. 2013;58:2082-2087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Hou LA, Laine L, Motamedi N, Sahakian A, Lane C, Buxbaum J. Optimal Timing of Endoscopic Retrograde Cholangiopancreatography in Acute Cholangitis. J Clin Gastroenterol. 2017;51:534-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Aboelsoud M, Siddique O, Morales A, Seol Y, Al-Qadi M. Early biliary drainage is associated with favourable outcomes in critically-ill patients with acute cholangitis. Prz Gastroenterol. 2018;13:16-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |